- Home
- Platforms
- EndureCARTTM: CAR-T Platform with Persistent Efficacy

CAR-T cell therapy is one of the most promising anti-cancer therapeutics, utilizing the body's immune system to combat cancer. However, challenges in solid tumor applications, such as the tumor microenvironment and cell heterogeneity, can diminish efficacy and potentially increase the toxicity of CAR-T therapy. To overcome these challenges, Protheragen has developed the EndureCARTTM technology platform. Through this innovative platform, we provide specialized services to clients in the CAR-T therapy sector, aiming to propel the advancement of the next generation of CAR-T therapies.
CAR-T cell therapy is an immunotherapy technique that genetically modifies T-cells to express chimeric antigen receptors (CARs) that identify and attach to specific antigens on cancer cells. These CARs consist of extracellular domains for target recognition and intracellular domains to activate T-cells against cancer cells. Once reintroduced into the individual, these engineered CAR-T cells can effectively locate and eliminate cancer cells, offering a promising approach to cancer therapeutic.
Limitations of Conventional CAR-T Therapy:
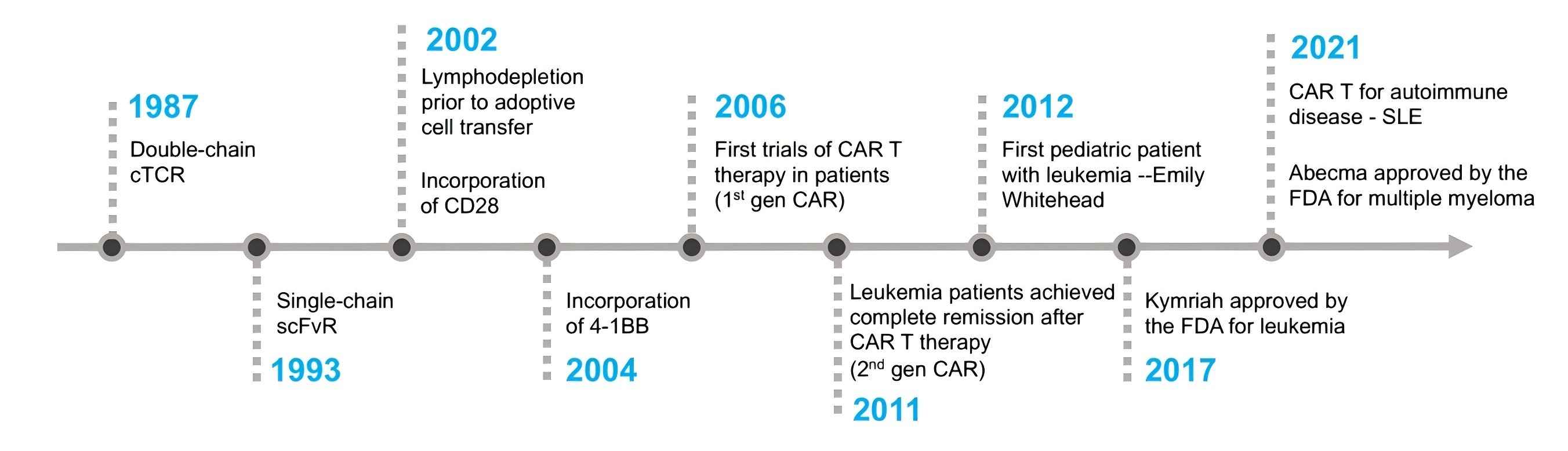 Fig.1 Significant milestones in the development of CAR-T cell therapy. (Mitra, A., et al., 2023)
Fig.1 Significant milestones in the development of CAR-T cell therapy. (Mitra, A., et al., 2023)
EndureCARTTM targets tumor metabolites or stromal proteins, recognizing the tumor microenvironment and activating T-cells sustainably by modifying the structure of chimeric antigen receptors, thus enhancing therapeutic efficacy. We have confirmed that CAR-T therapies targeting various targets, created using EndureCARTTM, exhibit enduring and effective anti-cancer properties.
The specific mechanism of action is that EndureCARTTM CAR-T cells with targeted effects penetrate tumor tissues, specifically recognize antigens, and trigger intracellular signaling pathways, prompting T-cell proliferation and cytokine release like IFN-1. This process reconstructs the tumor microenvironment, reversing immune suppression, and, with the collaboration of various immune cells, effectively eliminates tumor cells.
 Fig.2 Mechanism of EndureCARTTM CAR-T therapy.
Fig.2 Mechanism of EndureCARTTM CAR-T therapy.
The second-generation CAR comprises an activating domain and a co-stimulatory signaling domain like CD28 or 4-1BB. Leveraging the EndureCARTTM platform, we have developed an EDB-targeting CAR (EndureCAR-T) by fusing single-chain variable fragments to CD3ζ. This structure boosts downstream signaling mechanisms following CAR receptor activation, representing the fundamental advancement of EndureCARTTM.
This improved system can stimulate the STAT signaling pathway, with high-intensity JAK-STAT signaling activation dependent on tumor antigens, leading to continuous CAR cell proliferation and activation within tumor tissues. In contrast to the second-generation CAR-T cells, the EndureCAR enhances both T cell activation and the efficacy of tumor destruction in vitro and in vivo experiments.
Table. 1 Comparison of Conventional CAR-T with EndureCAR-T
| Types | Indicators | Conventional CAR-T | EndureCAR-T |
| In vitro testing | Tonic signal (No antigen exposure) | Elevated IFN-γ and IL-2 expression during the quiescent state | Lower tonic signal |
| Tumor cell lysis ratio (Repeated antigen exposure) | Upon repeated exposure to antigens, the cell lysis rate hovers around 45% | Despite repeated exposure to antigens, the cell lysis rate stays high at 82% | |
| Proliferation of CAR-T cells with repeated antigen stimulation | The fold change in cell numbers is roughly 1.35 | The fold change in cell numbers is around 2.6 | |
| In vivo evaluation | Efficacy validation | The tumor volume suppression effect is inadequate. (TGI: 50%) | Markedly suppressed tumor growth (TGI: 93%), and led to memory responses |
| Safety assessment | Approximately 10% weight loss | No detectable signs of toxicity. Minimal weight loss, around 1% |

Ensuring sustained T-cell activation for enduring therapeutic outcomes.
Boosting T-cell memory capabilities for persistent tumor cell elimination.
Maintaining high safety standards to reduce systemic toxicity and minimize therapeutic risks.
By collaborating with us, you can utilize our technology platform to achieve long-term activation of CAR-T cells, accelerating project progress. In addition, we provide comprehensive CAR-T therapy development services, spanning from target identification and CAR molecular design to in vitro and in vivo efficacy evaluation and safety assessment.
Our mission is to optimize CAR-T cell activation using advanced technology and innovative platforms, driving the evolution of innovative CAR-T therapeutics to enhance safety and efficacy. If you are interested in our technology platform and services, please contact us for further information.
Reference
For research use only, not for clinical use.