- Home
- Solutions
- By Diseases
- Hereditary Ophthalmic Diseases
- Diabetic Retinopathy (DR)

Diabetic retinopathy, or DR, is a pertinent complication of diabetes mellitus that threatens vision. Protheragen strives to promote innovation of diabetic retinopathy R&D. With our comprehensive diagnostic and therapeutics development services and preclinical research services, we are positioned in this area.
Diabetic retinopathy is divided into two phases: non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). During NPDR, initial microvascular changes take place that include the presence of microaneurysms, retinal hemorrhage, and intraretinal microvascular abnormalities (IRMA). While these changes do not exhibit symptoms, they can cause substantial visual loss if left untreated.
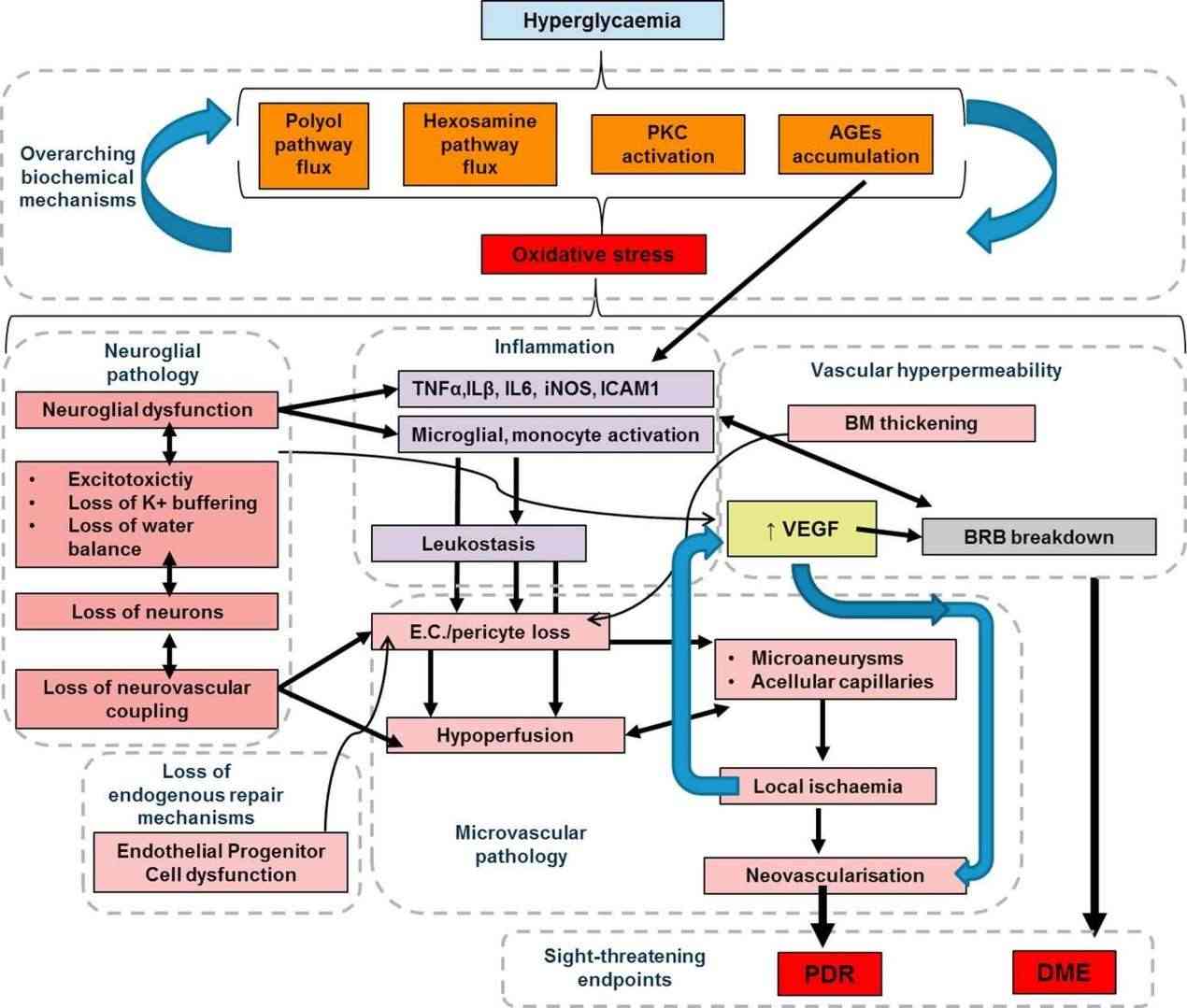 Fig.1 Pathogenic mechanisms leading to vision-threatening endpoints in diabetic retinopathy (DR). (Lechner J., et al., 2017)
Fig.1 Pathogenic mechanisms leading to vision-threatening endpoints in diabetic retinopathy (DR). (Lechner J., et al., 2017)The greater stage, PDR, is distinguished by neovascularization and is associated with other complications including vitreous hemorrhage and tractional retinal detachments. A common accompaniment of diabetic retinopathy is diabetic macular edema (DME) which is seen as a consequence of the disruption of the blood-retinal barrier (BRB) and subsequent fluid buildup within the macula ultimately culminating in loss of vision.
Table 1. Therapeutics for diabetic retinopathy. (Wang W., et al., 2018)
| Classification | Drugs | Status for DR Therapeutics | Clinical Benefits |
| Anti-VEGF | Pegaptanib (Macugen) | FDA approved | Greater BCVA improvement over sham groups in treating DME (phase 2/3, multicenter, two-year trial) |
| Aflibercept (EYLEA) | FDA approved | Greater BCVA improvement over laser in treating DME (VISTA, VIVID, DRCR.net Protocol T trials) and PDR (CLARITY trial) | |
| Non-specific anti-angiogenic | Squalamine (inhibits VEGF and other growth factors) | Phase 2 trial (clinicaltrials.gov ID: NCT02349516) in progress | - |
| AKB-9778 (Tie2 activator) | Phase 2 trial (clinicaltrials.gov ID: NCT01702441) in progress | Greater reduction in CRT in the combination group over the ranibizumab monotherapy group (phase 2a clinical trial) | |
| Intravitreal steroids | Triamcinolone | Off-label use | Greater improvements in triamcinolone + prompt laser group over laser alone in pseudophakic eyes |
| IL-6 inhibitor | EBI-031 (Section 3.2.2) | Clinical trial (clinicaltrials.gov ID: NCT02842541) in progress | - |
| Cardiolipin inhibitor | MTP-131 (OcuviaTM) | Clinical trial (clinicaltrials.gov ID: NCT02314299) in progress | - |
| Mitochondria specific antioxidant | ALA | Under clinical evaluation | Improved contrast sensitivity in type 1 and type 2 diabetes patients |
| Antioxidant | Lutein | Under clinical evaluation | Visual improvement in DR patients |
Disclaimer: Protheragen focuses on providing preclinical research services. This table is for information exchange purposes only. This table is not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
At Protheragen, we develop cutting-edge solutions for the therapeutic of diabetic retinopathy. Our range of activities covers everything from diagnostics to the development of new therapies, all within an advanced technological and scientific framework.



Protheragen excels in developing sophisticated animal models that accurately replicate the pathophysiology of DR. Our animal models include:

Pharmacologically Induced Models
Diet-Induced Models
Genetic Models
Environmental Exposure Models: Oxygen-Induced Retinopathy (OIR) Models
By leveraging our expertise in animal model development, Protheragen ensures that our clients have access to robust and relevant models for preclinical research. Our commitment to advancing DR therapeutics is reflected in our comprehensive suite of services, designed to support every stage of the development process. If you are interested in our services, please feel free to contact us.
References