- Home
- Solutions
- By Diseases
- Ocular Neoplastic Diseases
- Optic Nerve Glioma

Optic nerve gliomas (ONGs) are a group of low-grade gliomas that involve the optic nerve and associated visual structures, mainly in children. Protheragen provides full diagnostics and therapeutics development services for Optic Nerve Glioma using the latest technologies and skills in molecular biology, genomics, and drug development.
Optic nerve gliomas are tumours caused by optic pathway lesions that involve the optic nerve, chiasm, tracts, and radiations. These lesions are mostly low-grade astrocytomas whose growth and clinical presentation deviate significantly. ONGs are most frequently harmless in children; a sizeable population may remain asymptomatic for many years. Unfortunately, they may also grow extensively, resulting in some level of vision and other considerable neurological decline. Their association with NF1 worsens the clinical scenario as these tumours are more common at an earlier age, are often bilateral, and may lead to more severe complications. This specific understanding of ONGs is incredibly important for developing sufficient screening and therapeutics modalities for the condition.
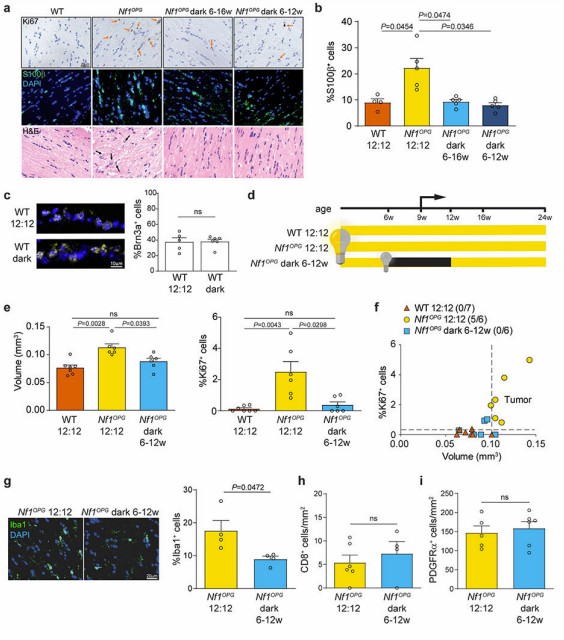 Fig.1 Retinal activity during a susceptible period is required for the initiation of Nf1-optic pathway gliomas (OPGs). (Pan Y., et al., 2021)
Fig.1 Retinal activity during a susceptible period is required for the initiation of Nf1-optic pathway gliomas (OPGs). (Pan Y., et al., 2021)The molecular and genetic profiling of ONGs has become more relevant over the last few years. Recently occurring specific genetic alterations along the NF1 gene (NB1 alterations) or BRAF rearrangements enable targeted therapies and personalised therapeutic options. Next-generation sequencing (NGS) and polymerase chain reaction (PCR) techniques can detect these mutations, offering valuable prognostic or therapeutic information. For instance, in some cancer types, the presence of BRAF mutations allows for the use of specific targeted inhibitors, which greatly enhances the effectiveness of the therapeutics.
| Therapeutics | Target | Description | Research Stage |
| Thioguanine/Procarbazine/CCNU/Vincristine (TPCV) | General Tumor Growth | This regimen shows a non-significant trend toward improved event-free survival compared to vincristine/carboplatin. However, it is associated with a risk of secondary leukemia and is generally avoided in patients with neurofibromatosis type 1 (NF1). | Approved |
| Cisplatin/Etoposide | General Tumor Growth | Achieves a 3-year progression-free survival rate of up to 78%. However, it carries risks of secondary leukemia and ototoxicity, limiting its use. | Approved |
| Temozolomide | General Tumor Growth | Used for progressive or refractory low-grade gliomas. It shows positive results with low toxicity but is generally avoided in NF1 cases. | Approved |
| Vinblastine | General Tumor Growth | Demonstrates efficacy in recurrent or refractory pediatric low-grade gliomas with low toxicity. | Approved |
| Vinorelbine | General Tumor Growth | Used in pediatric cases with progressive optic pathway gliomas, showing positive results. | Approved |
| Selumetinib (MEK Inhibitor) | MEK Pathway | Targets the MAPK pathway, showing a 2-year progression-free survival rate of up to 69%. It is particularly effective in patients with BRAF mutations. However, it may cause ocular toxicities such as the separation of outer retinal layers. | Phase II/III |
Disclaimer: Protheragen focuses on providing preclinical research services. This table is for information exchange purposes only. This table is not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
At Protheragen, we integrate the development of diagnostic and therapeutic solutions for optic nerve gliomas. This encompasses advanced techniques, molecular and genetic profiling, and even targeted therapies. Some of the specialised services we provide are:




Protheragen recognizes the unique needs of each research project and offers customized services to support the development of diagnostics and therapeutics for optic nerve gliomas. If you are interested in our services, please feel free to contact us.
References