Acromegaly
Acromegaly is a rare hormonal disorder caused by the overproduction of growth hormone (GH) by the pituitary gland. Our company excels in the field of rare diseases, including conditions like acromegaly, by offering comprehensive one-stop services catered to researchers and scientists.
Overview of Acromegaly
Acromegaly has a prevalence of approximately 60 per million. It is characterized by the enlargement of bones and tissues, leading to changes in facial features, enlarged hands and feet, joint pain, and other symptoms. Symptoms often develop slowly and can take years to become noticeable. If left untreated, acromegaly can have severe health implications, including an increased risk of cardiovascular disease, diabetes, and arthritis.
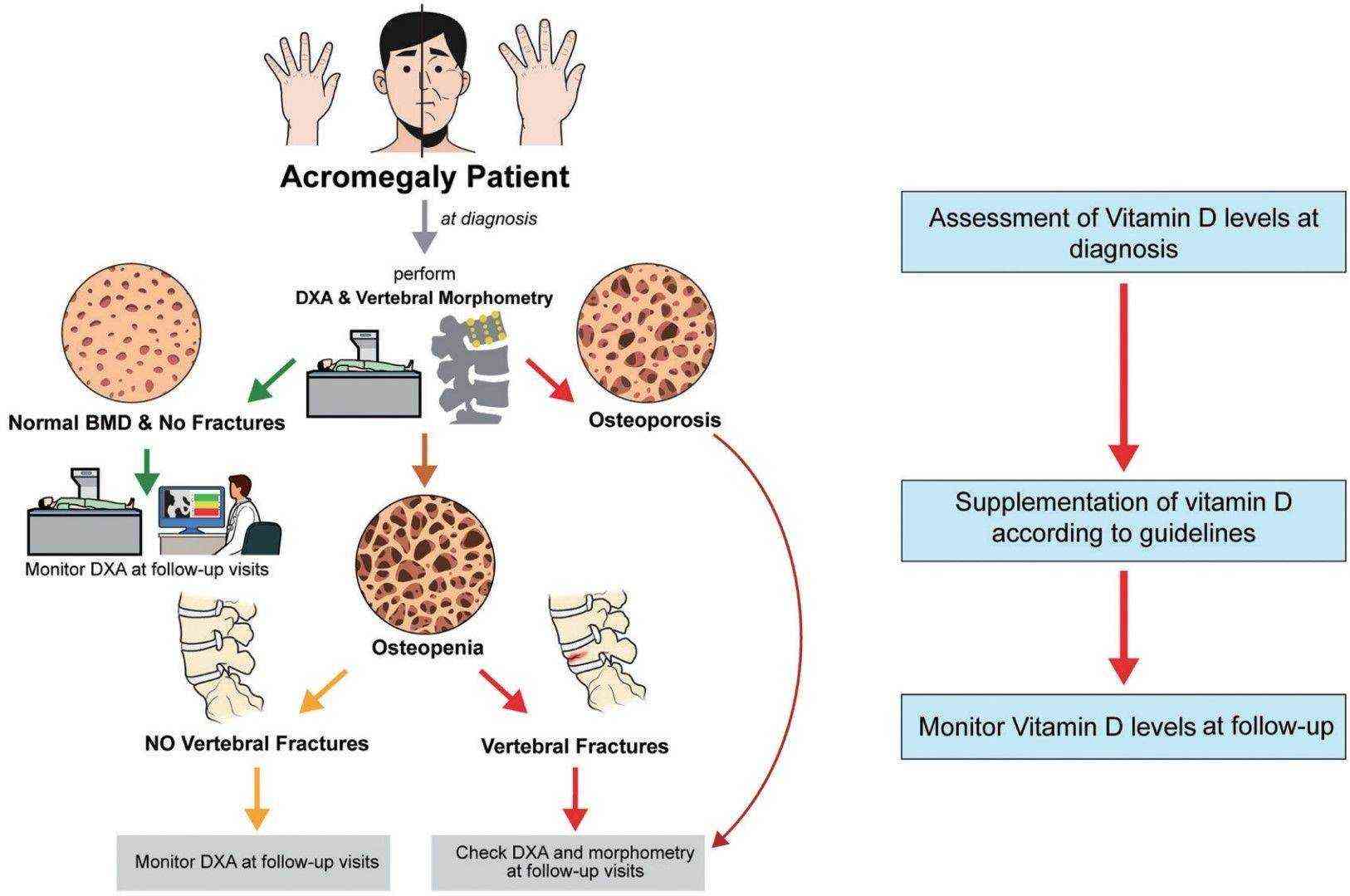 Fig.1 Proposed diagnostic and follow-up approach to bone comorbidities in acromegaly. (Giustina A., 2023)
Fig.1 Proposed diagnostic and follow-up approach to bone comorbidities in acromegaly. (Giustina A., 2023)Table 1 Genetics of Acromegaly.
| Condition or syndrome | Gene | Mutation type | Inheritance |
|---|---|---|---|
| Familial isolated pituitary adenoma | AIP | Inactivating | AD |
| Multiple endocrine neoplasia 1 | MEN1 | Inactivating | AD |
| Multiple endocrine neoplasia 4 | CDKN1B | Inactivating | AD |
| X-linked acrogigantism | GPR101 | Gene microduplication | X-linked |
| Hereditary paraganglioma-pheochromocytoma syndrome | SDHA-D, SDHAF2 | Inactivating | AD |
| McCune-Albright syndrome | GNAS | Somatic mosaicism; activating | None |
| Carney complex | PRKAR1A, PRKACB | Inactivating (PRKAR1A); activating (PRKACB) | AD |
| Neurofibromatosis 1 | NF1 | Inactivating | AD |
| Sporadic acromegaly | GNAS (gsp) | Somatic (tumor); activating | None |
Pathogenesis of Acromegaly
Acromegaly is typically caused by a benign tumor (pituitary adenoma) in the pituitary gland, leading to excess GH secretion. Excess GH stimulates the liver to produce insulin-like growth factor 1 (IGF-1), which promotes the growth of bones and tissues. In addition to its growth-promoting effects, excess GH can have metabolic effects, such as increasing insulin resistance, leading to glucose intolerance and a higher risk of developing diabetes mellitus.
 Fig.2 GH/IGF1 axis pathophysiology. (Danilowicz, K., and Sosa, S., 2023)
Fig.2 GH/IGF1 axis pathophysiology. (Danilowicz, K., and Sosa, S., 2023)Therapeutics Development of Acromegaly
| Types | Drug Names | Mechanism of Action | Targets | Research Phase |
|---|---|---|---|---|
| Somatostatin receptor ligands | Octreotide | SSTR agonists | SSTR | Approved |
| lanreotide | Achieve normal IGF-I | SSTR | Approved | |
| Pasireotide LAR | Normalize IGF-1 levels | SSTR | Phase III trials | |
| CAM2029 | Liquid crystal formulation of octreotide depot | SSTR | Phase III trials | |
| Other drugs | Cabergoline | D2 receptor agonist | D2 receptor | Approved |
| Pegvisomant | GHR antagonist | GHR | Approved | |
| Antisense oligonucleotide | ATL1103 | Target the mRNA encoding the GHR | GHR | Phase II trials |
Our Services
Our team of experienced professionals is dedicated to collaborating with researchers, offering guidance, consultation, and customized solutions to accelerate discoveries in the field of rare diseases. Our animal models and therapeutic development platform, enable us to provide specialized support throughout the research process.
Therapeutics Development Platforms
Animal Models of Acromegaly
Animal models of acromegaly play a crucial role in understanding the pathophysiology and testing potential therapeutics. Our company offers a variety of animal models for researchers seeking to gain a deeper understanding of the mechanisms underlying acromegaly and to assess the efficacy of novel therapy approaches.

Genetic engineering animal models involve altering the genetic makeup of animals to mimic specific aspects of the disorder, such as manipulating the genes responsible for regulating GH production.
Optional Models: AipGt(RRI002)Byg model; Gpr101em1Cya model, etc.
Why Choose Us
By providing a seamless integration of services including pharmacokinetic study and drug safety evaluation, we empower researchers and scientists to make significant strides in understanding, diagnosing, and treating conditions like acromegaly.
If you are interested in exploring our services further, we invite you to contact us for additional details and personalized quotations.
References
- Danilowicz, Karina, and Soledad Sosa. "Acromegaly and Cancer: An Update." Archives of medical research 54.8 (2023): 102914.
- Ershadinia, Nazanin, and Nicholas A Tritos. "Diagnosis and Treatment of Acromegaly: An Update." Mayo Clinic proceedings 97.2 (2022): 333-346.
- Giustina, Andrea. "Acromegaly and Bone: An Update." Endocrinology and metabolism (Seoul, Korea) 38.6 (2023): 655-666.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
