In Vitro Permeability and Transporter Studies
In vitro permeability and transporter studies can influence pharmacokinetic, pharmacodynamic, and toxicological properties. Our company employs experienced scientists who focus on every aspect of rare disease drug development and research, and can carry out entire in vitro studies to evaluate the permeability and transporter of drug candidates.
Permeability of Drugs
Permeability refers to the rate at which drug molecules can permeate through a biological membrane barrier. It is a necessary process for a drug to be absorbed in the small intestine, to permeate the blood-organ barriers, to permeate into cells, and to be cleared by the liver and kidneys. It is also one of the determinant factors affecting the oral bioavailability of drugs, as well as their absorption through the intestinal tract. Therefore, permeability studies of compounds play an important role in the early stages of drug development.
Transporters of Drugs
Transporters are a series of proteins that exist on the surface of cell membranes and are responsible for the transmembrane transport of drugs. Transporters can affect the in vivo processes of drugs, such as absorption, distribution, and elimination, thereby affecting the effectiveness and safety of drugs, and play an important role in drug-drug interactions.
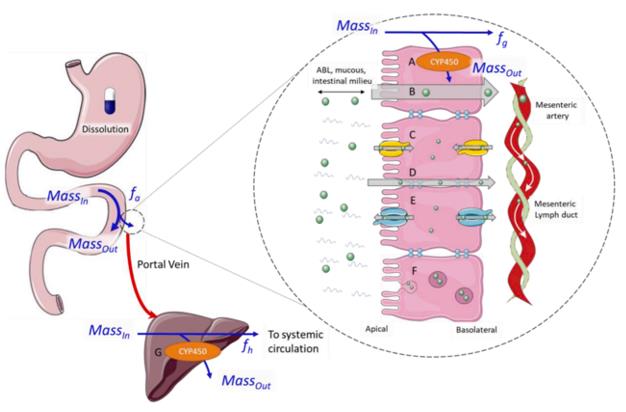
Fig.1 Schematic diagram of the absorption process of oral drugs from the gastrointestinal tract. (O'Shea, J P., et al., 2022)
The initial goal of most drug research and development projects is to develop oral drugs, and oral drugs must go through the processes of ADME after entering the human body. The study of in vitro permeability and transporters is an important part of the drug research and development process. It plays an active role in ADME research and accelerates the development of rare disease drugs.
Our Services
Our company has established a complete in vitro ADME detection system, which has been widely used in the selection of candidate compounds during the development process of rare disease new drug projects.
- Parallel Permeability Assay
- MDR1-MDCKII Permeability Assay
- Wild-type MDCKII Permeability Assay
- Caco-2 Permeability Assay
- Transporters: BCRP, P-gp, OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, OATP2B1, PEPT1/2, ASBT, NTCP MATE1, and MATE2-K
- Hepatocyte Uptake Experiments
- Substrate and Inhibition Assay Formats
- Initial Screening Assessments or Definitive Determinations
Our Advantages
- Professional team with many years of experience in permeability and transporter studies
- Competitive pricing and fast turnaround time
- Careful design and transparent operation process
- High data quality and reliable analysis
- Exceptional post-sales support
Project Workflow
With a research team with extensive expertise in pharmacokinetics, our company is confident to provide customers with in vitro permeability and transporter studies services for rare diseases. We customize personalized solutions and analysis processes according to your different scientific research needs and track the progress of projects in real time to accelerate your scientific discovery. If you are interested in our service, please contact us for more details.
Reference
- O'Shea, J P., et al., "Best practices in current models mimicking drug permeability in the gastrointestinal tract-An UNGAP review." European Journal of Pharmaceutical Sciences, 170 (2022): 106098.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
