Organoid Models Development Service
The development of organoid models can provide important information for drug research and development, the establishment of disease models, precision healthcare, and regenerative healthcare at the organ level. Our company has long-term devoted to the development and application of organoid models. We are pleased to use our extensive experience and one-stop technology platforms to offer the best service to satisfy each demand of our customers in rare disease research.
Overview of Organoid Models
Organoids refer to the use of adult stem cells (ASC) or organs pluripotent stem cells (PSC) in vitro self-assemble into 3D organ-type structures, which are very similar to the histological characteristics of human organs and can reproduce the physiological function of organs in vitro. Organoids have the characteristics of cell proliferation, differentiation, self-renewal, self-assembly, and long-term culture, and are an important bridge between traditional 2D culture and in vivo mouse models. Compared with traditional 2D culture models, organoids have many advantages, such as being closer to physiological cell composition and behavior, more stable genome, and more suitable for biological transfection and high-throughput screening.
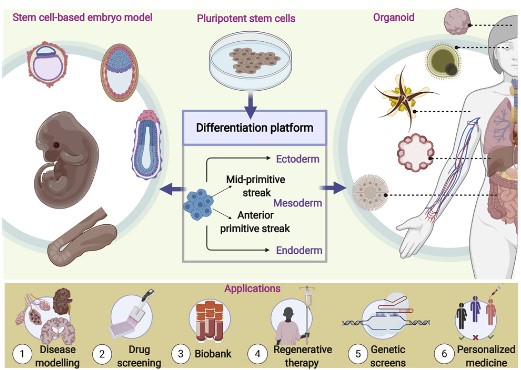 Fig.1 Different differentiation platforms and organoid applications. (Sahu, S., and Sharan, S. K., 2020)
Fig.1 Different differentiation platforms and organoid applications. (Sahu, S., and Sharan, S. K., 2020)
Organoid Models for Rare Diseases
Rare diseases pose significant challenges in terms of understanding their underlying mechanisms and developing effective therapeutics. Traditional research approaches often rely on animal models or cell lines, but these may not fully capture the complexity and nuances of rare diseases in humans. However, recent advancements in the field of organoid models have provided promising avenues for studying and advancing our knowledge of rare diseases.
Table 1 Organoid models for rare diseases. (Heydari, Zahra, et al., 2021)
| Tissue/organ | Rare Disease | Cell source | Outcomes and achievements | Matrix |
|---|---|---|---|---|
| Brain | Parkinson's disease | iPSC | An organoid-based model is presented that recapitulates the pathological features of lrrk2 associated with sporadic PD, which contributes to the progression of therapeutic discovery. | Matrigel embedding |
| Retina | Late-onset retinitis pigmentosa | - | The first phenotypically consistent late-onset retinitis pigmentosa model was established. This provides insight into the PDE6B-related mechanisms of RP. | Matrigel suspension |
| Skin | Atopic eczema | - | This study elucidates the mechanism by which FLG haploinsufficiency leads to atopic skin inflammation, contributing to understanding filaggrin-deficient phenotypes and the underlying molecular mechanisms associated with atopic skin inflammation. | - |
| Cardiac | Barth syndrome | iPSC | Organoids replicate the pathophysiology of Barth syndrome cardiomyopathy. This model provides new ideas for studying the pathogenesis of Barth syndrome and opens up new avenues for the therapeutics of Barth syndrome. | - |
| Dilated cardiomyopathy | - | Engineered models show sarcomere dysfunction and impaired responses to mechanical and beta-adrenergic stress. This suggests that titin mutations cause dilated cardiomyopathy by disrupting the link between myogenesis and adaptive remodeling. | Collagen solution | |
| Duchenne muscular dystrophy | iPSC | In Duchenne muscular dystrophy-engineered cardiac organoids, we observed restoration of proper contractile function. This model provides a powerful tool for eliminating genetic causes and correcting muscle abnormalities. | Collagen solution | |
| Kidney | Polycystic kidney disease | iPSC | This model recapitulates glomerular disease associated with podocyte epithelial injury and is proven to be a replicable and versatile model in regenerative healthcare. | Matrigel sandwich |
| Autosomal recessive polycystic kidney disease | iPSC | Patient-derived hiPSC mutant organoids exhibit a cystic phenotype that can be effectively prevented through gene correction or drug therapy. The discovery provides new avenues for studying kidney development and drug discovery. | - | |
| Congenital nephrotic syndrome | - | The expression of PODOCIN and NEPHRIN is reduced in the glomerular organoid model, providing a feasible method for screening podocyte toxicity. | - | |
| Lung | Idiopathic pulmonary fibrosis | iPSC-MSC | The model faithfully reproduced IPF for drug screening. | Alginate beads |
| Liver | Wolman disease | Patient-derived iPSCs | Therapeutics of FGF19 in the culture system resulted in improved survival rates and reductions in lipid accumulation, ROS production, and tissue stiffness. | Matrigel embedding |
| Alagille syndrome | - | Structural abnormalities in the biliary tree were successfully replicated in the simulation. | Matrigel embedding | |
| Colon and intestine | Hirschsprung disease | PSCs | The model investigated the cellular and molecular mechanisms underlying Hirschsprung disease caused by a mutation in the PHOX2B gene. | Matrigel embedding |
Our Services
With the world's leading 3D culture technology, our company has successfully cultured a variety of organoid types such as lungs, stomach, intestines, liver, kidneys, etc. Our technical team provides comprehensive and customized organoid model development services to simulate the occurrence, progression, and therapeutic response of rare diseases, thereby accelerating the research and development of your rare disease therapies.
- Source of Organoids
- Adult stem cell (ASC) source
- Embryonic stem cell (ESC) source
- Induced pluripotent stem cell (iPSC) source
- Patient-derived organoid (POD)
- Types of Organoids
- Brain organoid
- Inner ear organoid
- Retinal organoid
- Skin organoid
- Cardiac organoid
- Kidney organoid
- Lung organoid
- Liver organoid
- Pancreatic organoid
- Gastric organoid
- Intestinal organoid
- Etc.
- Applications of Organoids
- Drug screening by organoid models
- Toxicity testing by organoid models
- Efficacy evaluation by organoid models
- Organ-on-a-Chip
Why Choose Us?
As an integrated CRO, our company has extensive expertise in organoid model development for rare disease research. Whatever your specific requirements, we will work closely with you to develop a customized solution. We can quickly respond to the changing needs of your rare disease research projects. If you are interested in our services, please feel free to contact us for more details and quotation information of related services.
References
- Sahu, S., and Sharan, S. K. "Translating embryogenesis to generate organoids: Novel approaches to personalized medicine." Iscience 23.9 (2020).
- Heydari, Zahra, et al. "Organoids: a novel modality in disease modeling." Bio-design and Manufacturing 4 (2021): 689-716.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
