Recombinant Protein Development Platform
Recombinant proteins have different biological activities and are widely used in different fields of biological research and development. Our company provides comprehensive services for biopharmaceutical companies and scientific research institutions to develop recombinant proteins for rare diseases. Our recombinant protein development platform offers multiple advantages, including expertise, customization, high-quality products, fast turnaround times, competitive pricing, confidentiality, and regulatory compliance.
Introduction to Recombinant Proteins
Recombinant protein production is a protein obtained by applying recombinant DNA or recombinant RNA technology. The production of recombinant proteins in vitro mainly includes four major systems: E. coli expression system, mammalian expression system, yeast expression system, and insect expression system. Choose an appropriate protein expression system based on your downstream application to improve the expression success rate. Recombinant proteins can be divided into the following types according to their functions:
- Interleukin (IL)
- Interferon (IFN)
- Tumor necrosis factor (TNF)
- Colony stimulating factor (CSF)
- Growth factor (GF)
- Chemokine
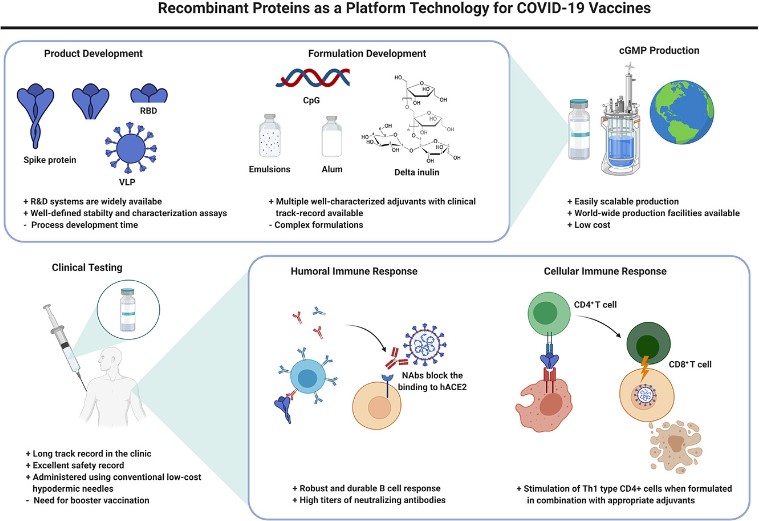
Fig.1 Application examples of recombinant proteins. (Pollet, J., et al., 2021)
Applications of Recombinant Proteins
Based on different expression systems, recombinant proteins are used in different fields:
- Prophylactic recombinant protein vaccine
- Development of diagnostic reagents
- Enzyme preparation
- Immune checkpoints
- Antibody-drug targets
- CAR-T cell therapy targets
- Fc receptors
- Influenza viral proteins
- Cytokines
- Immunodetection reagents
- Protein based drugs
Our Services
With the introduction of top technical talents and the increase of cooperation projects, our company's recombinant protein development platform has been greatly improved and built. We have the ability to provide recombinant protein production such as enzymes, transmembrane proteins, viral antigens, kinases, etc. From gene synthesis, and vector construction to protein expression, purification, and modification, we provide a one-stop recombinant protein development service for your rare disease research.
| Services | Process | Cycle |
|---|---|---|
| E. coli Expression System | Gene synthesis and codon optimization | 1-2 weeks |
| Vector construction | 1-2 weeks | |
| Protein expression | 1-2 weeks | |
| Flask fermentation and protein purification | 2-3 weeks | |
| Yeast Expression System | Gene synthesis and codon optimization | 1-2 weeks |
| Vector construction | 1-2 weeks | |
| Electrotransformation | 1-2 weeks | |
| Screening of high-copy strains | 1 week | |
| Protein expression | 2-3 weeks | |
| Protein purification and QC analysis | 2-3 weeks | |
| Mammalian Expression System | Gene synthesis and codon optimization | 1-2 weeks |
| Vector construction | 1-2 weeks | |
| Transient transfection of HEK293/CHO cells | 1-2 weeks | |
| Protein purification and QC analysis | 2-3 weeks | |
| Insect Expression System | Gene synthesis and codon optimization | 1-2 weeks |
| Vector construction | 1-2 weeks | |
| Virus packaging and amplification | 2-3 weeks | |
| Cell transfection and expression detection | 3-4 weeks | |
| Protein expression | 2-3 weeks | |
| Protein purification and QC analysis | 1-2 weeks | |
| VLP-based Membrane Protein Expression | Gene synthesis and codon optimization | 1-2 weeks |
| Vector construction | 1-2 weeks | |
| Transient transfection of HEK293 cells | 1-2 weeks | |
| VLP protein purification and QC analysis | 2-3 weeks | |
| Inclusion Body Protein Renaturation | Vector construction | 1-2 weeks |
| Protein purification | 1-2 weeks | |
| Small-scale protein renaturation | 2-3 weeks | |
| Mass protein production | 2-4 weeks |
Project Workflow

With a research team with extensive expertise in therapeutic protein development, our company is confident to provide clients with recombinant protein development services for rare diseases. We have the capabilities and resources to provide professional communication and problem-solving support to ensure that we can quickly respond to the changing needs of your rare disease therapy research projects. If you are interested in our services, please feel free to contact us for more details and quotation information of related services.
Reference
- Pollet, J., et al. "Recombinant protein vaccines, a proven approach against coronavirus pandemics." Advanced drug delivery reviews 170 (2021): 71-82.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
