Alstrom Syndrome (ALMS)
Alstrom syndrome is an uncommon genetic condition characterized by a broad range of symptoms impacting multiple organ systems. Typically, this disorder presents with abnormalities in vision and hearing. Specialized drug and therapy development services are essential to enhance and expedite Alstrom syndrome research. Our company is well-equipped to address your drug and therapy development requirements in Alstrom syndrome therapy.
Introduction to Alstrom Syndrome
Alström syndrome is a very rare, autosomal recessive genetic disorder that affects multiple systems within the body. This condition is characterized by a variety of symptoms including vision and hearing impairments, obesity, insulin resistance, type 2 diabetes, and cardiomyopathy. It is caused by mutations in the ALMS1 gene located on chromosome 2p13. The prevalence of Alström syndrome is extremely low, estimated at about 1–9 cases per million people. This syndrome can vary significantly in terms of symptom onset and severity, affecting individuals differently depending on the specific genetic mutations they carry.
 Fig. 1 ALMS1 gene (top) and ALMS1 protein. (bottom). (Alvarez-Satta, M., et al., 2015)
Fig. 1 ALMS1 gene (top) and ALMS1 protein. (bottom). (Alvarez-Satta, M., et al., 2015)Pathogenesis of Alstrom Syndrome
Alstrom syndrome, a rare genetic disorder, emerges due to mutations in the ALMS1 gene. This mutation disrupts the function of the primary cilium, a cell organelle crucial for intracellular and intercellular sensing and signaling. In Alström syndrome, the dysfunction of the primary cilium leads to a broad range of symptoms, including obesity, type 2 diabetes mellitus (T2DM), and various organ dysfunctions. The pathogenesis of these symptoms is linked to altered pathways in insulin signaling and β-cell function, highlighting the central role of the primary cilium in metabolic regulation.
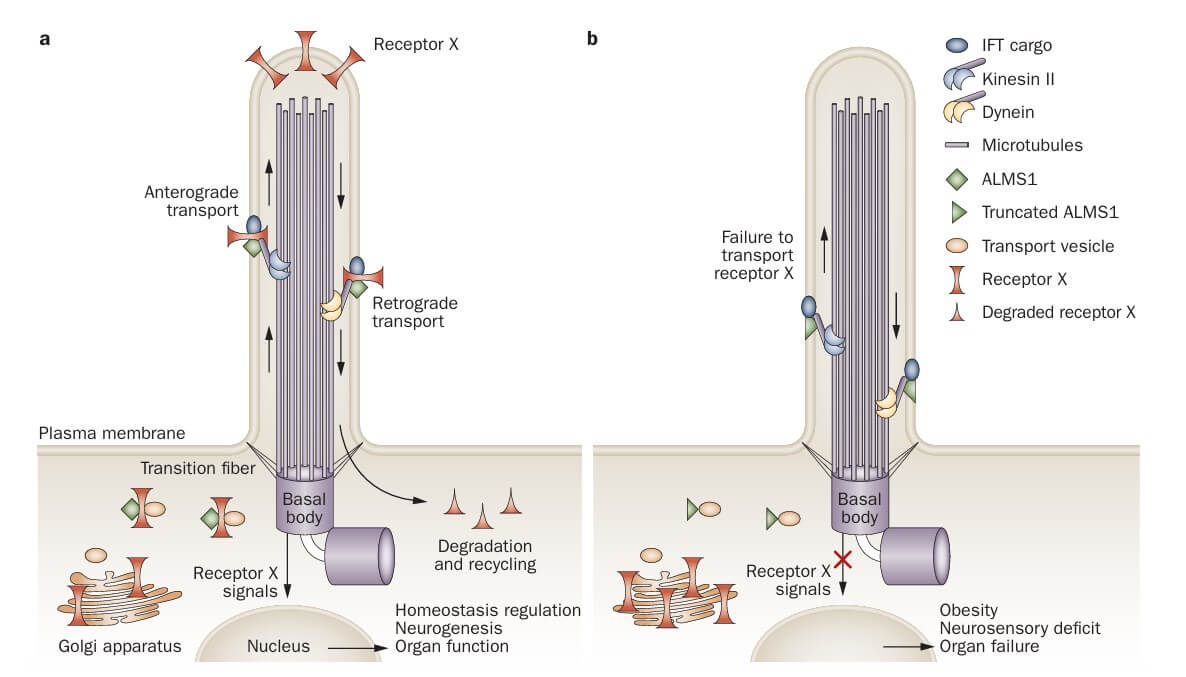 Fig. 1 Postulated function of ALMS1 protein. (Girard, D. and Petrovsky, N., 2011)
Fig. 1 Postulated function of ALMS1 protein. (Girard, D. and Petrovsky, N., 2011)Diagnosis Development of Alstrom Syndrome
Genetic analysis techniques like next-generation sequencing (NGS), which include methods such as targeted gene panels and whole-exome sequencing (WES), have facilitated detailed genetic examinations. It has been found that mutations in the ALMS1 gene, situated on chromosome 2p13, are responsible for Alstrom syndrome in most instances.
Therapy Development of Alstrom Syndrome
Small molecule drugs offer targeted interventions by modulating specific pathways or molecular mechanisms implicated in Alström syndrome. These drugs include metabolic modulators like insulin sensitizers and lipid-lowering agents, as well as protein kinase inhibitors and compounds targeting ciliary function.
Monoclonal antibodies offer highly specific targeting of disease-related molecules or cells. They may be utilized as anti-inflammatory agents to mitigate complications like cardiomyopathy or insulin resistance, or as neuroprotective agents to preserve vision and hearing function by protecting retinal photoreceptors or auditory hair cells.
Stem cell therapy, utilizing mesenchymal stem cells or induced pluripotent stem cells, aims to replace damaged cells in tissues such as the retina, heart, or pancreas. Additionally, gene-modified cells could potentially express therapeutic proteins or correct underlying genetic defects associated with Alstrom syndrome.
Gene replacement therapy aims to restore normal gene function by introducing functional copies of the ALMS1 gene using viral vectors. Alternatively, genome editing technologies could be employed to precisely modify disease-causing mutations in the ALMS1 gene or other relevant genes.
Our Services
Our company adopts a partnership-driven approach. We collaborate closely with clients to craft tailored, innovative Alstrom syndrome therapy strategies and ensure robust support throughout the process.
Platforms of Alstrom Syndrome Therapy Development
Animal Models of Alstrom Syndrome
We have established expertise in developing and utilizing relevant animal models that closely mimic the disease characteristics and response to therapy. These models enable us to evaluate the safety and efficacy of potential therapies.
| Non-Genetically Engineering Models | ||
| We provide diverse model choices customized to meet specific research needs related to Alstrom syndrome. These models allow researchers to simulate and study the complex biological processes associated with Alstrom syndrome. | ||
| Special Diet Induced Models | ||
| Optional Models |
|
|
| Chemical Induced Models | ||
| Optional Models |
|
|
| Genetically Engineered Models | ||
| Our expertise in genetic engineering techniques, allows us to generate accurate and reliable models that recapitulate the genetic alterations observed in human Alstrom syndrome. | ||
| Optional Models |
|
|
| Optional Species | Mice, Rats, Non-human primates, Others | |
In addition to these models, our comprehensive services encompass other models that target specific signaling pathways and molecular targets.
If our services align with your goals, please contact us for more details.
References
- Alvarez-Satta, M., et al., "Alstrom syndrome: current perspectives." Appl Clin Genet, (2015). 8: p. 171-179..
- Girard, D. and Petrovsky, N., "Alstrom syndrome: insights into the pathogenesis of metabolic disorders." Nature Reviews Endocrinology, (2011). 7(2): p. 77-88.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
