Crigler-Najjar Syndrome
Crigler-Najjar syndrome is a rare genetic disorder characterized by high levels of unconjugated bilirubin in the blood, a product of heme degradation. Our company stands out as a leading entity in the realm of rare diseases like Crigler-Najjar syndrome, offering one-stop comprehensive services that cater specifically to researchers in this field.
Overview of Crigler-Najjar Syndrome
This condition affects approximately 0.6 to 1 in 1 million newborns globally, making it a challenging area of study. Two distinct types of Crigler-Najjar syndrome vary in severity and prognosis.
- Type 1: This is the more severe form of the condition where there is a complete absence of the enzyme responsible for processing bilirubin. Without therapy, individuals with type 1 Crigler-Najjar syndrome can develop severe jaundice, brain damage, and other complications.
- Type 2: This form is less severe than type 1 because there is some enzyme activity (less than 10%) present, although it is insufficient to process bilirubin adequately. Individuals with type 2 Crigler-Najjar syndrome typically have milder symptoms and a better prognosis than those with type 1.
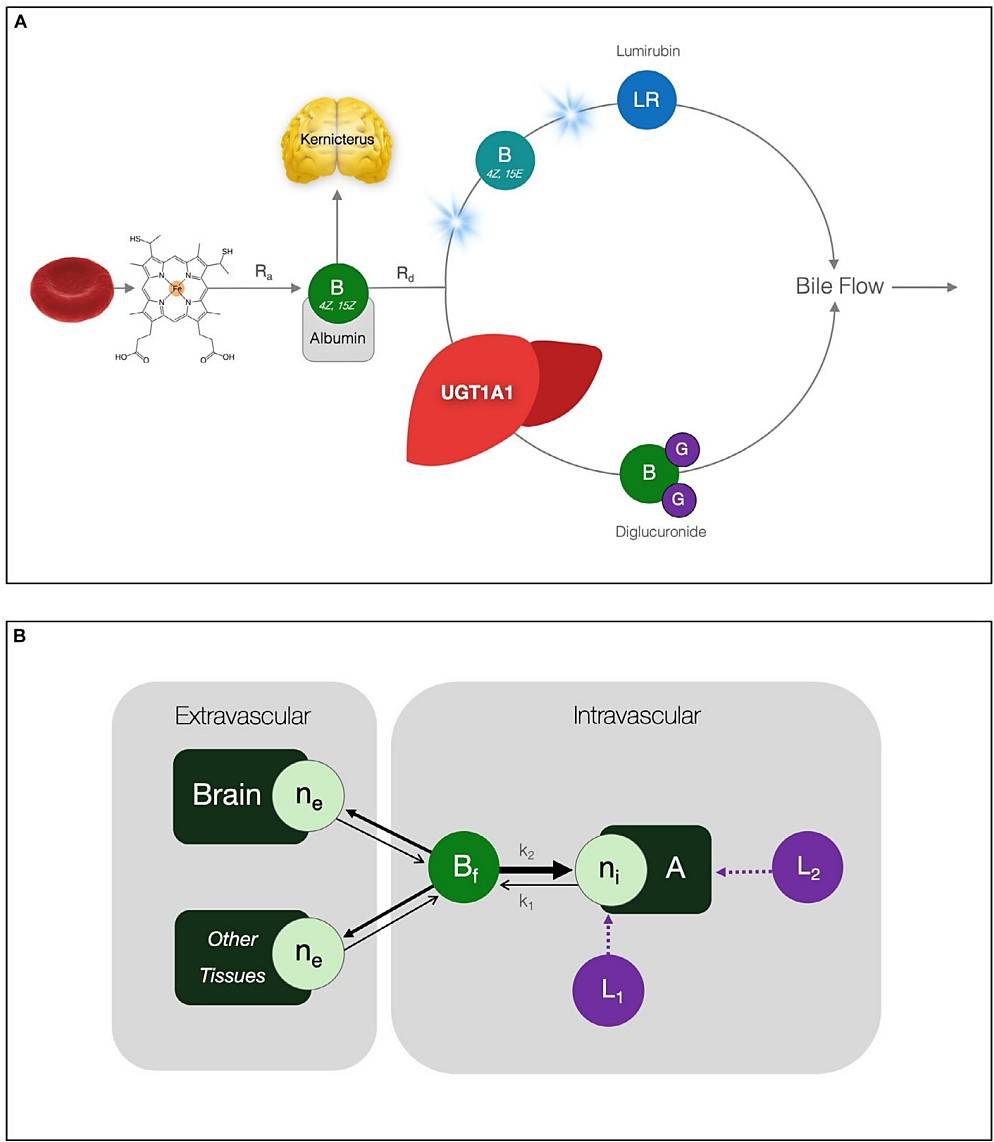 Fig.1 Bilirubin metabolism and biodistribution. (Strauss, K. A., et al., 2020)
Fig.1 Bilirubin metabolism and biodistribution. (Strauss, K. A., et al., 2020)Pathogenesis of Crigler-Najjar Syndrome
The underlying cause of Crigler-Najjar syndrome is a deficiency in the enzyme UDP-glucuronosyltransferase 1A1 (UGT1A1) responsible for conjugating bilirubin, leading to the accumulation of unconjugated bilirubin in the blood. High levels of unconjugated bilirubin can cross the blood-brain barrier and cause neurological damage, resulting in symptoms such as kernicterus (bilirubin-induced brain damage).
 Fig.2 General strategy of AAV8-mediated gene editing in the Ugt1a gene in combination with the CRISPR-SaCas9 platform. (Bortolussi, G., et al., 2023)
Fig.2 General strategy of AAV8-mediated gene editing in the Ugt1a gene in combination with the CRISPR-SaCas9 platform. (Bortolussi, G., et al., 2023)
Therapeutics Development of Crigler-Najjar Syndrome
| Types | Drug Names | Mechanism of Action | Targets | Research Phase |
|---|---|---|---|---|
| Small molecule drugs | Phenobarbital | Enzyme-inducing agents | GABAA receptor | Approved |
| Calcium phosphate | Bilirubin-binding agents | / | Phase III trials | |
| Ursodeoxycholic acid | A cholesterol inhibitor, apoptosis inhibitor, and FXR antagonist | FXR | Phase IV trials | |
| Tin-protoporphyrin | Heme-oxygenase inhibitors | HMOX1 | Phase III trials | |
| Cell therapy | HepaStem | Restore hepatic enzyme activity | / | Phase I/II trials |
| Gene therapy | GNT0003 | Correct the dysfunction of a mutated gene | UGT1A1 | Phase I/II trials |
| LNP-encapsulated hUGT1A1 mRNA | Restore bilirubin levels to normal | UGT1A1 | Preclinical research |
Our Services
Our integrated services encompass everything from access to a diverse network of experts and resources, specialized laboratory facilities, and cutting-edge technologies tailored for rare disease research. Our animal model and therapeutic development platform enable researchers to efficiently collaborate, innovate, and make substantial advancements in understanding and treating conditions like Crigler-Najjar syndrome.
Therapeutics Development Platforms
Animal Models of Crigler-Najjar Syndrome
Animal models are crucial for studying diseases like Crigler-Najjar syndrome to better understand the underlying mechanisms and develop potential therapeutics. Our company provides various animal models to provide valuable insights into the pathophysiology of the condition, enabling researchers to develop novel therapeutic strategies.

Chemical-induced animal models of Crigler-Najjar syndrome involve the administration of certain compounds or chemicals to animals to mimic the symptoms and characteristics of the human disease.
Optional Models: Bilirubin-induced model; Chemical toxins-induced model, etc.

The genetic engineering animal models are engineered to have mutations in the gene responsible for bilirubin conjugation, resulting in elevated levels of unconjugated bilirubin similar to the human condition.
Optional Models: UGT1 knockout model; Gunn rat model, etc.
Why Choose Us
We provide one-stop services including pharmacokinetic research and biosafety evaluation, and our commitment to supporting researchers in rare diseases is unwavering, ensuring that they have the necessary tools and support to make meaningful contributions to this crucial area of study.
If you are interested in our services, do not hesitate to reach out to us for more information about the services we provide.
References
- Strauss, Kevin A et al. "Crigler-Najjar Syndrome Type 1: Pathophysiology, Natural History, and Therapeutic Frontier." Hepatology (Baltimore, Md.) 71.6 (2020): 1923-1939.
- Bortolussi, Giulia et al. "CRISPR-Cas9-mediated somatic correction of a one-base deletion in the Ugt1a gene ameliorates hyperbilirubinemia in Crigler-Najjar syndrome mice." Molecular therapy. Methods & clinical development 31 (2023): 101161.
- Tcaciuc, Eugen et al. "Management of Crigler-Najjar syndrome." Medicine and pharmacy reports 94 (2021): S64-S67.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
