Methylmalonic Acidemia (MMA)
Methylmalonic and propionic acidemia (MMA/PA) are two disorders on the same metabolic pathway that are relatively common among organic acid metabolic diseases. These conditions can easily lead to severe metabolic acidosis, hyperammonemia, coma, and even death. At our company, we've assembled a team of seasoned professionals with a wealth of experience in navigating drug and therapy development challenges specific to Methylmalonic Acidemia.
Introduction to Methylmalonic Acidemia
Methylmalonic acidemia is a rare genetic disorder caused by defects in the enzyme methylmalonyl-CoA mutase or in the metabolic pathway of its cofactor, vitamin B12. This enzyme deficiency leads to the accumulation of methylmalonic acid and other toxic compounds in the body, resulting in metabolic crises and multi-organ complications. The disease manifests in infancy, often with symptoms such as lethargy, vomiting, hypotonia, and developmental delays. The prevalence of MMA is estimated to be between 1 in 48,000 to 1 in 100,000 live births worldwide.
 Fig. 1 Manifestations of methylmalonic acidemia. (Head, P.E., et al., 2023)
Fig. 1 Manifestations of methylmalonic acidemia. (Head, P.E., et al., 2023)Pathogenesis of Methylmalonic Acidemia
Methylmalonic acidemia is primarily caused by a deficiency in the enzyme methylmalonyl-CoA mutase, leading to the accumulation of methylmalonic acid in the body. This enzyme defect can result from mutations in the MMUT gene or from defects in the cobalamin (vitamin B12) metabolic pathway. Individuals with severe forms of the disease (mut0) present symptoms early in life, show no response to vitamin B12 supplementation, and have a high mortality rate. The disease severity is associated with the enzymatic activity level and response to B12 supplementation, influencing the management and prognosis of the individuals.
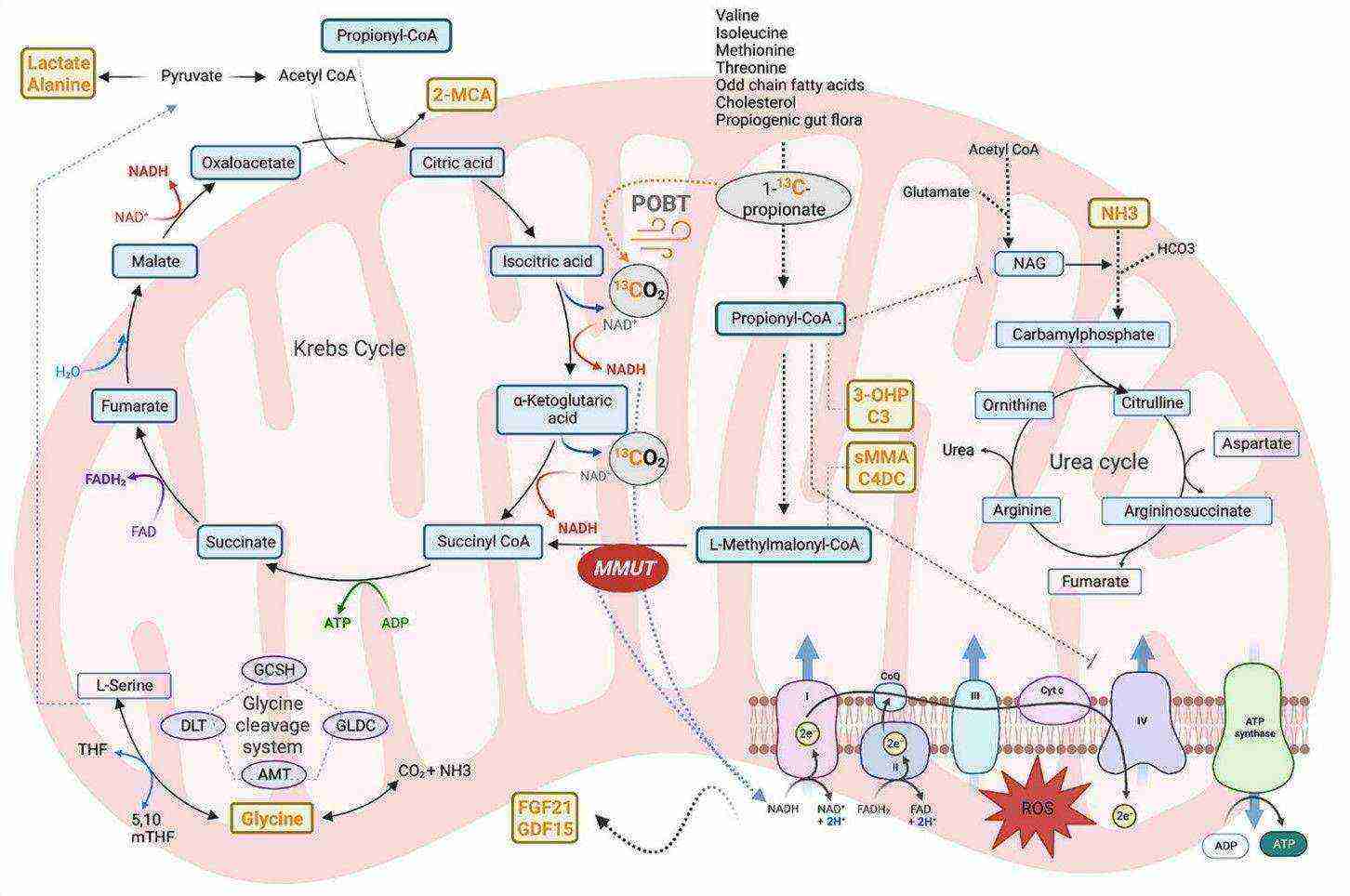 Fig. 2 The main biochemical pathways involved in isolated methylmalonic acidemia. (Manoli, I., et al., 2023)
Fig. 2 The main biochemical pathways involved in isolated methylmalonic acidemia. (Manoli, I., et al., 2023)Biomarkers of Methylmalonic Acidemia
- Methylmalonic Acid (MMA): Serum, cerebrospinal fluid, and urine levels of methylmalonic acid are the hallmark biomarkers for MMA, accumulating in all forms of the disorder.
- 2-Methylcitric Acid (2-MCA) and 3-Hydroxypropionic Acid (3-OHP): These metabolites accumulate in MMA and propionic acidemia due to the condensation of propionyl-CoA with oxaloacetate in the TCA cycle. They serve as diagnostic and monitoring biomarkers.
- Fibroblast Growth Factor-21 (FGF21) and Growth Differentiation Factor-15 (GDF15): These biomarkers reflect mitochondrial dysfunction and kidney injury. Their concentrations are significantly elevated in individuals with severe mut0 and cblB-type MMA, correlating with decreased propionate oxidation capacity.
- Propionylcarnitine (C3): Elevated plasma concentrations of propionylcarnitine, along with variable elevations of methylmalonyl/succinylcarnitine (C4DC) and the ratios of C3/C2 or acyl/free carnitine, form the basis for newborn screening for MMA.
Gene Therapy of Methylmalonic Acidemia
Table1. Gene therapy experiments in murine models of methylmalonic acidemia.
| Vector | MMA Murine Model Tested (Phenotype) | Promoter | cDNA/mRNA | Outcomes |
|---|---|---|---|---|
| Ad5 | Mmut−/− (neonatal lethal) | CMV | Mmut | Increased survival, decreased pMMA |
| Lentivirus (HIV-1) | Mmut−/−MMUTh2 (hypomorph) | EF1α | Codon-optimized MMUT mRNA | Increased survival, decreased pMMA |
| rAAV8 | Mmut−/− (neonatal lethal) | CBA | Mmut | Increased survival, decreased pMMA |
| rAAV9/rAAV2 | Mmut−/− (neonatal lethal) | CBA | Codon-optimized MMUT | Increased survival, decreased pMMA |
| rAnc80/rAAV8 | Mmut−/−; TgINS-MCK-Mmut (hypomorph) | hAAT | Codon-optimized MMUT | Improved growth, decreased pMMA |
| rAAV8 | Mut−/−; TgINS-MCK-Mmut (hypomorph) | CBA | Codon-optimized MMUT | Improved growth, decreased pMMA, decreased Fgf21 |
| rAAV-DJ | Mmut−/− (neonatal lethal)Mut−/−; TgINS-MCK-Mmut (hypomorph) | Promoterless GeneRide™ | Codon-optimized MMUT | Increased survival, decreased pMMA |
| rAAV44.9 | Mmut−/− (neonatal lethal)Mmut−/−; TgINS-MCK-Mmut (hypomorph) | CBA | MMUT | Increased survival, improved growth, decreased pMMA |
| rAnc80 SVP-Rapa | Mmut−/−; TgINS-MCK-Mmut (hypomorph) | hAAT | MMUT | Improved growth, decreased pMMA, repeat-dosing |
| LNP | Mmut−/−; TgINS-MCK-Mmut (hypomorph) | None | Codon-optimized MMUT mRNA | Improved growth, decreased pMMA |
Our Services
Our company adopts a collaborative approach, partnering closely with clients to create tailored and innovative therapy strategies for Methylmalonic Acidemia. We offer comprehensive support throughout the entire development process, ensuring effective and customized solutions.
Platforms of Methylmalonic Acidemia Therapy Development
Animal Models of Methylmalonic Acidemia
We have considerable expertise in developing and employing animal models that accurately replicate the disease characteristics and therapeutic responses seen in Methylmalonic Acidemia. These models are instrumental in investigating the underlying mechanisms and assessing the safety and effectiveness of prospective therapies with precision.
| Non-Genetically Engineering Models | ||
| Our company offers a range of models specifically designed to meet the unique research needs of Methylmalonic Acidemia. These models enable researchers to simulate the disease and delve deeply into its pathogenesis and therapeutic development. | ||
| Optional Models |
|
|
| Genetically Engineered Models | ||
| Our expertise in genetic engineering techniques, enables us to create precise and reliable models to recreate the disease in various model organisms. | ||
| Optional Models |
|
|
| Optional Species | Mice, Rats, Non-human primates, Others | |
Moreover, we provide a variety of detailed animal model services focused on particular signaling pathways and molecular targets.
If you are interested in our services, please don't hesitate to contact us.
References
- Head, P.E., et al., "New insights into the pathophysiology of methylmalonic acidemia." J Inherit Metab Dis, (2023). 46(3): p. 436-449.
- Manoli, I., et al., "Biomarkers to predict disease progression and therapeutic response in isolated methylmalonic acidemia." J Inherit Metab Dis, (2023). 46(4): p. 554-572.
- Chandler, R.J. and Venditti, C.P., "Gene Therapy for Methylmalonic Acidemia: Past, Present, and Future." Hum Gene Ther, (2019). 30(10): p. 1236-1244.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
