Paroxysmal Nocturnal Hemoglobinuria (PNH)
Paroxysmal nocturnal hemoglobinuria (PNH) is a disease of clonal stem cells marked by the destruction of red blood cells within the blood vessels, caused by the immune system's complement component targeting these cells. Specialized drug and therapy development services are essential to enhance and expedite PNH research. Our company is well-equipped to address your drug and therapy development requirements in PNH therapy.
Introduction to PNH
PNH is a rare, acquired disorder characterized by intravascular hemolysis and hemoglobinuria. Leukopenia, thrombocytopenia, venous and arterial thrombosis, and paroxysmal episodes are common manifestations of this condition. The estimated incidence of PNH is around one per million, with a prevalence of about eight per million. Roughly 20–35% of individuals with PNH are expected to die within 5–10 years after being diagnosed, even with the best supportive care available.
Pathogenesis of PNH
The primary defect in PNH is a somatic mutation in the PIGA gene. This mutation results in the loss of glycosylphosphatidylinositol (GPI) anchors and the proteins they carry on hematopoietic cells. The deficiency of GPI-anchored proteins like CD55 (Decay Accelerating Factor, DAF) and CD59 (Membrane Inhibitor of Reactive Lysis, MIRL) is central to the disease's mechanism. In the absence of these protective proteins, the complement system is triggered excessively on the surfaces of cells, leading to the formation of membrane attack complexes. This triggers intravascular hemolysis of susceptible red blood cells and activates PNH leukocytes and platelets. These events heighten the risk of thrombosis and systemic illness, often resulting in premature death.
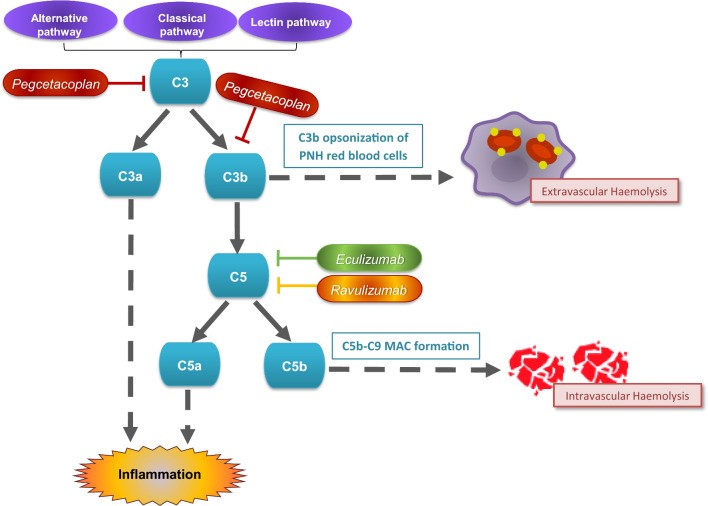 Fig.1 Mechanism of action of complement inhibitors in paroxysmal nocturnal haemoglobinuria. (Heo, Y.A., 2022)
Fig.1 Mechanism of action of complement inhibitors in paroxysmal nocturnal haemoglobinuria. (Heo, Y.A., 2022)Diagnostic Development of PNH
Flow cytometry, a widely used diagnostic method, analyzes the expression of specific membrane proteins (such as CD55 and CD59) on red blood cells to detect PNH. Genetic testing has gained importance, focusing on mutations in the PIGA gene, located on the X chromosome, offering enhanced diagnostic accuracy. Additionally, exploring novel biomarkers with increased specificity and sensitivity aids in early diagnosis and monitoring of disease progression. Integrating these approaches improves the diagnostic precision of PNH.
Therapy Development of PNH
Small molecule drugs target specific pathways involved in the disease mechanism of PNH. Examples include Eculizumab and Ravulizumab, both complement inhibitors targeting the C5 protein. Factor D inhibitors are in trials to target early complement pathway components.
Cell therapies for PNH focus on restoring healthy bone marrow function. Hematopoietic stem cell transplantation (HSCT) is currently the only curative therapy for PNH. In addition, mesenchymal stem cell therapy is being explored to support marrow engraftment and reduce graft-versus-host disease in transplant settings.
Eculizumab is the first mAb used in PNH therapy. Recently, pegcetacoplan was approved, which blocks complement protein C3, offering an alternative mechanism of action compared to C5 inhibitors. This broadens the options for individuals who may not respond adequately to traditional therapies.
Gene therapy represents a futuristic but rapidly advancing field in treating PNH. The goal is to correct the underlying genetic defects, specifically mutations in the PIGA gene. Techniques like CRISPR/Cas9 and other gene-editing technologies can potentially correct these mutations in hematopoietic stem cells directly.
Our Services
At our company, we offer a comprehensive range of services to support TA diagnostics and therapy development. By analyzing PNH's genetic and molecular characteristics, we can identify specific biomarkersand tailor therapy strategies accordingly.
Platforms of PNH Therapy Development
Animal Models of PNH
We offer animal model services to assess the efficacy and safety of potential therapies to support your research efforts. Utilizing our advanced facilities and expertise, we aim to expedite the translation of promising therapeutic approaches from the laboratory to application.
| Non-Genetically Engineering Models | ||
| Our company specializes in delivering top-notch services to create NON-GEMs. We provide diverse model choices customized to meet specific research needs related to PNH. | ||
| Optional Models |
|
|
| Genetically Engineered Models | ||
| Our expertise in genetic engineering techniques, allows us to generate accurate and reliable models that recapitulate the genetic alterations observed in human PNH. | ||
| Optional Models |
|
|
| Optional Species | Mice, Rats, Non-human primates, Others | |
In addition to these models, our wide-ranging services include other models that focus on PNH's particular signaling pathways and specific molecular targets.
If our services resonate with you, we invite you to contact us whenever works best for you. Let's discuss customizing our solutions to seamlessly align with your objectives and aspirations.
References
- Heo, Y.A., "Pegcetacoplan: A Review in Paroxysmal Nocturnal Haemoglobinuria." Drugs, (2022). 82(18): p. 1727-1735.
- Bravo-Perez, C., et al., "Paroxysmal Nocturnal Hemoglobinuria: Biology and Treatment." Medicina (Kaunas), (2023). 59(9).
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
