Drug safety evaluation is fundamental to ensuring the therapeutic efficacy and patient well-being in rare skin disease treatment development. Our comprehensive safety assessment platform combines advanced in vitro and in vivo models with specialized expertise in dermal toxicology. Our integrated approach encompasses both local skin reactions and systemic safety profiles, enabling informed decision-making throughout the drug development process.
Introduction to Rare Skin Diseases Drug Safety Evaluation
Drug safety evaluation for rare skin diseases presents unique challenges and considerations in pharmaceutical development. These evaluations require specialized approaches due to the complex nature of rare dermatological conditions and their distinct pathological mechanisms. Safety assessments must account for both topical effects at the application site and potential systemic implications, particularly given the skin's role as a barrier and its interaction with the immune system.
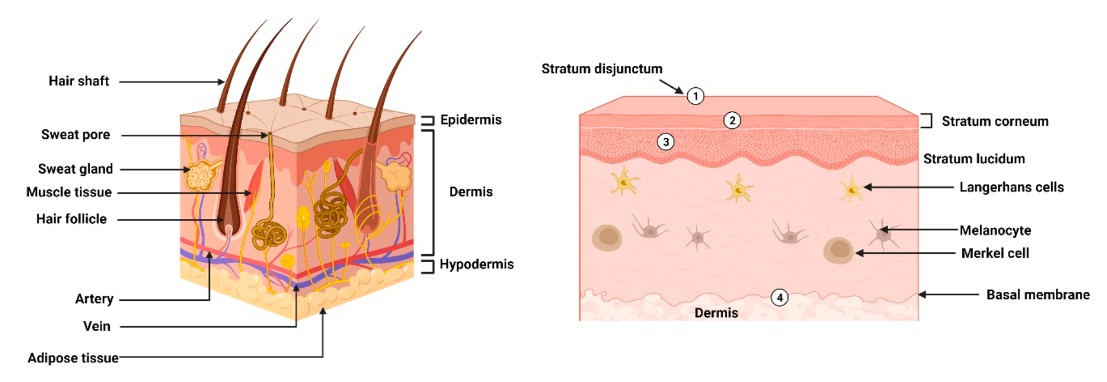
Fig.1 Morphology and physical barriers in the epidermis layers of skin. (Raina, N.,
et al., 2023)
Methods for Drug Safety Evaluation of Rare Skin Diseases
In the development of therapies for rare skin diseases, a comprehensive safety evaluation framework is essential. This framework includes in vitro toxicity testing, histopathological analysis and dermatopharmacokinetic studies. These methods ensure thorough risk assessment and safe therapeutic interventions.
In Vitro Toxicity Testing:
Utilizing cultured skin cells and 3D skin models to assess cytotoxicity, irritation, and allergic potential of new drugs, providing initial safety data without animal testing.
Histopathological Analysis:
Examining tissue samples from animal models to identify any pathological changes or skin damage resulting from drug application, helping to anticipate adverse effects in humans.
Dermatopharmacokinetic Studies:
Studying drug kinetics and accumulation within skin layers to predict safe dosage levels, minimizing systemic exposure and potential toxicity.
Key Aspects of Safety Evaluation for Rare Skin Diseases
Safety evaluation for therapies targeting rare skin diseases focuses on several crucial aspects
- Local Tissue Response and Skin Barrier Function: Assessing how a drug affects the skin's structural integrity and barrier capabilities, ensuring it does not compromise these essential protective functions.
- Immune Response and Sensitization Potential: Evaluating the likelihood of immune reactions and allergic responses that a drug may elicit, thus preventing adverse impacts on immune health.
- Drug Absorption and Distribution Patterns: Analyzing how a drug penetrates and spreads within skin layers to ensure it reaches target areas effectively without causing systemic exposure and toxicity.
- Long-term Safety Considerations for Chronic Conditions: Ensuring that therapies are safe for prolonged use, particularly in treating persistent or chronic rare skin conditions.
- Special Attention to Genetic and Autoimmune Skin Disorders: Recognizing the unique safety challenges posed by hereditary and autoimmune skin diseases, tailoring safety assessments to address these specific risks.
Our Services
Our company offers comprehensive, one-stop preclinical services to support the development of rare disease therapies. Our expertise spans disease model development, providing tailored models that closely mimic human conditions; drug metabolism and pharmacokinetics (DMPK), offering precise evaluations of drug behavior in biological systems; and drug safety evaluation, ensuring rigorous assessments for safety and efficacy. With a focus on rare diseases, we deliver high-quality, reliable data to accelerate and strengthen your therapeutic pipeline.
Drug Safety Evaluation Services
We are committed to providing expert safety evaluation services for rare skin diseases. Our team of skilled toxicologists, immunologists, and researchers employs advanced methodologies to assess critical aspects such as local tissue responses, immune system reactions, and long-term safety for chronic conditions.
Why Choose Us?
Professional core technical team.
Advanced experimental equipment.
Empowering success through cooperation.
Strict quality control system.
At our company, we are committed to providing comprehensive, one-stop preclinical development services that cover every aspect from disease model creation to innovative therapy research. If you are interested in our services, please don't hesitate to contact us.
References
- Raina, N., et al. "New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective." ACS Omega 8.22 (2023): 19145-67.
- Suarez, E. A., et al. "Novel Methods for Pregnancy Drug Safety Surveillance in the Fda Sentinel System." Pharmacoepidemiol Drug Saf 32.2 (2023): 126-36.
All of our services and products are intended for preclinical research use
only and cannot be used to diagnose, treat or manage patients.


 Fig.1 Morphology and physical barriers in the epidermis layers of skin. (Raina, N., et al., 2023)
Fig.1 Morphology and physical barriers in the epidermis layers of skin. (Raina, N., et al., 2023)