Tularemia
Tularemia is a febrile illness caused by the Gram-negative bacterium Francisella tularensis, similar to typhoid fever. It is characterized by primary local ulcerative lesions, regional lymphadenopathy, and systemic symptoms and occasionally presents as atypical pneumonia. Specialized drug and therapy services are essential to enhance and expedite Tularemia research. Our company is well-equipped to address your drug and therapy development requirements in Tularemia therapy.
Introduction to Tularemia
Tularemia, caused by the bacterium Francisella tularensis, is recognized for its potential resurgence in areas affected by war or natural disasters due to its ability to thrive under deteriorating sanitary conditions. Globally, the incidence of tularemia is generally low but varies significantly by region and local environmental conditions, influenced by factors such as wildlife reservoirs and human interaction with affected ecosystems. Between 1992 and 2012, 18343 human cases were reported to the WHO-CISID or ECDC databases.
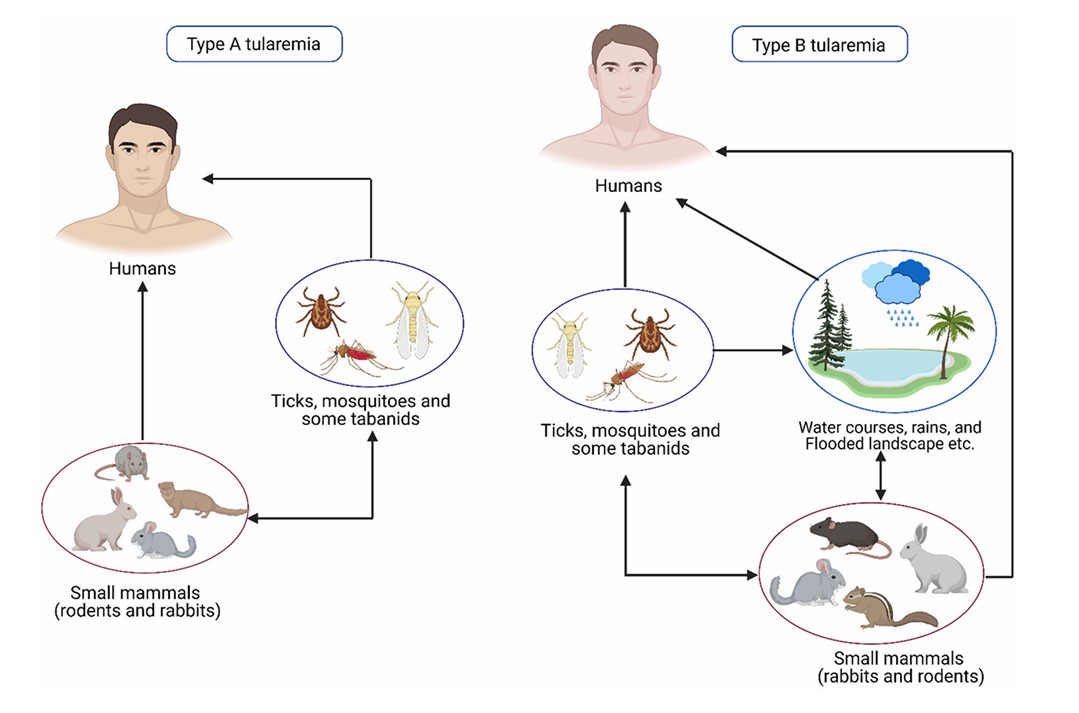 Fig.1 Modes of transmission of tularemia. (Sharma, R., et al., 2023)
Fig.1 Modes of transmission of tularemia. (Sharma, R., et al., 2023)Pathogenesis of Tularemia
Francisella tularensis can infect various host cells, including macrophages, dendritic cells, neutrophils, hepatocytes, endothelial cells, and type II lung epithelial cells. Following its engulfment by these cells, the bacterium swiftly breaks out from the phagosome into the cytoplasm, where it proliferates, ultimately causing the host cell to undergo apoptosis. This process releases the bacteria, facilitating further infection and spread throughout the body. It adeptly avoids immune detection and can multiply extensively in vital organs such as the liver, spleen, and lungs until the immune system mounts a response that includes a damaging cytokine storm.
 Fig.2 Pathogenesis of tularemia. (Sharma, R., et al., 2023)
Fig.2 Pathogenesis of tularemia. (Sharma, R., et al., 2023)Diagnostics Development of Tularemia
Francisella tularensis is underway, alongside the development of new immunological assays that surpass traditional serological tests in specificity and sensitivity. Additionally, the introduction of molecular techniques, especially polymerase chain reaction (PCR), has significantly transformed the diagnostic landscape for tularemia. PCR enables the rapid and precise detection of Francisella tularensis DNA in clinical samples, offering much quicker results than older culture methods. These advancements are particularly crucial for early diagnosis and efficient outbreak management.
Therapy Development of Tularemia
These compounds target specific pathways involved in bacterial survival and proliferation. For instance, small molecules can inhibit critical enzymes or block signaling pathways that Francisella tularensis utilizes for infection, thus halting the bacteria's ability to cause disease.
Although more common in cancer therapy, cell therapies could be used in infectious diseases like tularemia by engineering immune cells to better recognize and destroy bacteria. This could involve modifying T cells or other immune cells to enhance their antibacterial activities.
These are designed to target specific antigens found on the surface of Francisella tularensis. By binding to these antigens, monoclonal antibodies can neutralize the bacteria or mark them for destruction by other immune cells. This method provides a targeted approach to strengthen the immune response against the infection.
In the context of tularemia, gene therapies might involve delivering genetic material into individual cells to produce proteins that inhibit bacterial growth or enhance the immune response to the infection. This approach could offer a way to boost the innate and adaptive immune responses more effectively.
Our Services
Our company adopts a partnership-driven approach. We collaborate closely with clients to craft tailored, innovative Tularemia therapy strategies and ensure robust support throughout the process.
Platforms of Tularemia Therapy Development
Animal Models of Tularemia
We have established expertise in developing and utilizing relevant animal models that closely mimic the disease characteristics and response to therapy. These models enable us to evaluate the safety and efficacy of potential therapies.
| Non-Genetically Engineering Models | ||
| Non-genetically engineered animal models of tularemia mainly include naturally occurring or experimentally induced infections in suitable animals without genetic modification. We provide diverse model choices customized to meet specific research needs related to Tularemia. | ||
| Optional Models |
|
|
| Genetically Engineered Models | ||
| Our expertise in genetic engineering techniques, allows us to generate accurate and reliable models that recapitulate the genetic alterations observed in human Tularemia. | ||
| Optional Models |
|
|
| Optional Species | Mice, Rats, Non-human primates, Others | |
In addition to these models, our comprehensive services encompass other models that target specific signaling pathways and molecular targets.
If our services align with your goals, please contact us for more details.
References
- Sharma, R., et al., "Tularemia - a re-emerging disease with growing concern." Vet Q, (2023). 43(1): p. 1-16.
- Maurin, M., et al., "Tularemia treatment: experimental and clinical data." Front Microbiol, (2023). 14: p. 1348323.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
