In Vitro ADME Services
In the early stages of drug discovery, in vitro ADME studies (absorption, distribution, metabolism, and excretion) are critical in understanding candidate compounds' efficacy and safety. Our company has highly experienced and matured scientists capable of undertaking the entire set of services from drug discovery to pre-clinical studies to accelerate your ADME studies on the treatment of rare diseases.
Introduction to ADME
In vitro ADME studies are used to evaluate the absorption, distribution, metabolism, and elimination of drugs. In the early stages of drug discovery, in vitro ADME studies are used to identify chemotypes and lead compounds with favorable pharmacokinetic and toxicological profiles.
During the lead optimization and preclinical candidate stages, comprehensive in vitro ADME studies including protein binding, metabolic stability, drug interaction-related transporters, and CYP inhibition are often required to predict human pharmacokinetic properties. Optimizing the properties of ADME in drug design and screening can significantly reduce the failure rate of drug discovery. With the realization of new technologies and the improvement of existing technologies, high-throughput screening technology is widely used in ADME studies.
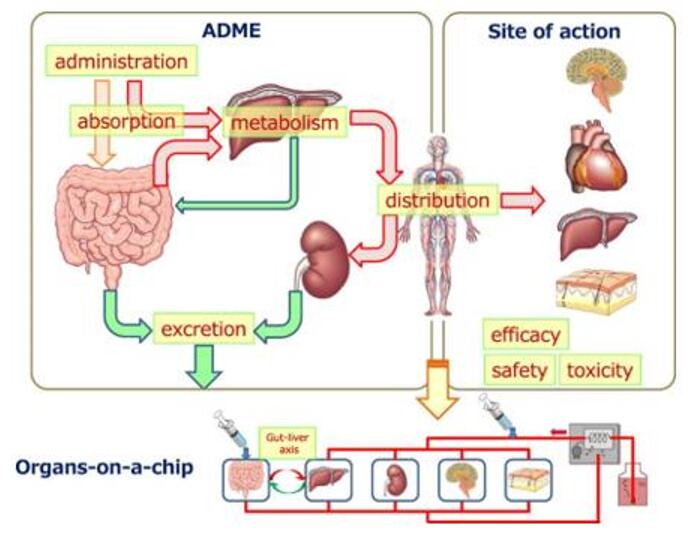
Fig.1 Research and application of in vitro ADME. (Ishida, Seiichi, 2018)
ADME studies can enable scientists to obtain more candidate molecule information in the drug discovery of rare diseases. In vitro ADME studies improve the efficiency of the development of drugs for rare diseases.
What Services Can We Provide?
The physiochemical studies services we can provide include but are not limited to: blood partitioning, chemical stability, Kinetic / thermodynamic solubility, lipophilicity (LogD & LogP), pKa, and buffer stability.
Our company has extensive expertise in vitro metabolism, our professional team can provide services including cytochrome P450 studies, stability studies, protein binding studies, etc.
In Vitro Permeability and Transporter Studies
A complete permeability and transporter studies system can be used in the selection of candidate compounds during the development process of rare disease new drug projects.
Our Advantages
- Providing standardized upstream platform solutions
- Competitive pricing and fast turnaround time
- Superior data quality and fast turnaround
- Careful design and transparent operation process
- High-quality one-stop service
Project Workflow

With many years of experience and a proven track record of quality, innovation, and customer support in rare disease research, our company provides comprehensive services for in vitro ADME studies to leading and emerging biopharmaceutical companies and scientific research institutions. Please feel free to contact us and send your detailed request.
Reference
- Ishida, Seiichi. "Organs-on-a-chip: current applications and consideration points for in vitro ADME-Tox studies." Drug metabolism and pharmacokinetics 33.1 (2018): 49-54.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
