In Vivo Pharmacokinetics Services
In vivo pharmacokinetics is mainly used to study the preclinical pharmacokinetics of drugs in experimental animals. Our company has extensive expertise in pharmacokinetics and can provide efficient methods to accelerate your rare disease therapy development. We can provide a one-stop, personalized, and customized service according to your specific needs.
Introduction to In Vivo Pharmacokinetics
Pharmacokinetics (PK) is dedicated to determining the absorption mechanism, biological distribution, and elimination of the drug from the body. After entering the body, drugs are absorbed into the bloodstream and transported across through biological membranes to target tissues to bind to receptors and produce a pharmacological effect. Once the effect has ended, the drug must be eliminated from the body. In vivo pharmacokinetic studies provide necessary and useful information for this, such as steady-state distribution, clearance, half-life, AUC, bioavailability, and tissue distribution, etc., to help understand the efficacy or toxicity of the drug in the body and provide more opportunities to discover more biologically and safer therapeutic drugs.
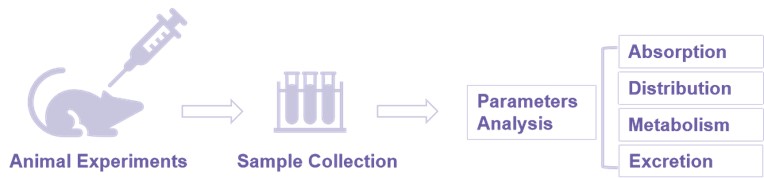 Fig.1 Typical study process of in vivo pharmacokinetics.
Fig.1 Typical study process of in vivo pharmacokinetics. Our Services
Our company has been keeping up with market demand, constantly innovating, and has established a complete animal model library to support in vivo pharmacokinetic studies. We can provide rare disease drug/therapeutic development services and can support the development of small molecules, proteins, antibodies, cell therapies, nucleic acid drugs, etc., and in vivo pharmacokinetic services.
- Oral Drug Absorption
- Dermal Absorption
- Other Non-Oral Drug Absorption
Quantitative Tissue Distribution Studies
- Blood-brain Barrier Studies
- Tissue Half-life Studies
- Target Engagement Extent Studies
- Excretion Route and Rate Studies
- Biological Sample Analysis
- Experimental Design and Execution
- Data Analysis of Bioavailability Studies
Metabolite Profiling and Identification Studies
- Structure Elucidation of Metabolites
- Studies of The Metabolic Pathways
- Determination of Relative Abundance of Metabolites
- Mass Balance Studies in Animal Models
- Radioactive Analysis of Standard Matrices
- Radioactive Analysis of Alternative Matrices
- Biliary Excretion Studies in Animal Models
- Biliary Radioactive Analysis
- Biliary Metabolite Identification
- Enterohepatic Circulation Studies
Our company also provides in vitro ADME studies to meet all your requirements for pharmacokinetic service's needs, and we have the capabilities and resources to provide professional communication and problem-solving support to ensure that we can quickly respond to the changing needs of your rare disease research projects.
Our Advantages
- The rich experience accumulated in successful cases of new drug development
- Timely project reporting and after-sales service
- Fast and cost-efficient workflow
- Careful design and transparent operation process
- High data quality and reliable analysis
Project Workflow

Our company's in vivo pharmacokinetics services are a simplified workflow by a group of specially trained and experienced scientists. We customize personalized solutions and analysis processes according to your different scientific research needs and track the progress of projects in real time to accelerate your rare disease therapy research and development. If you are interested in our services, please feel free to contact us for more information.
All of our services and products are intended for preclinical research use only and cannot be used to diagnose, treat or manage patients.
